Abstract

Background/Introduction: Advanced-stage MZL is generally considered incurable, characterized by periods of remission and relapse. Zanubrutinib (BGB-3111) is a potent and highly specific next-generation Bruton tyrosine kinase (BTK) inhibitor recently approved by the US Food and Drug Administration and Health Canada for the treatment of patients (pts) with R/R MZL based on the primary analysis results of the MAGNOLIA study (BGB-3111-214; NCT03846427). Here, we present the final analysis of MAGNOLIA at a median follow-up of 28 months.
Methods: MAGNOLIA is a phase 2, multicenter, single-arm study of adult pts requiring systemic treatment for R/R MZL with ≥1 prior line of therapy including ≥1 CD20-directed regimen. All pts were treated with zanubrutinib 160 mg twice daily until disease progression or unacceptable toxicity. Use of long-term antiplatelets and anticoagulants was permitted. The primary endpoint was overall response rate (ORR) as determined by an independent review committee (IRC) in accordance with the Lugano classification. Secondary endpoints included ORR by investigator assessment, duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety. Efficacy was assessed by positron emission tomography (PET)-based Lugano criteria for pts with IRC-confirmed fluorodeoxyglucose (FDG)-avid disease at baseline; non-avid pts were assessed by computed tomography (CT)-based criteria. A sensitivity analysis using only CT-based criteria was also performed.
Results: As of May 4, 2022, 68 pts were enrolled and treated. The median age was 70 years (range 37-95), with 27.9% aged ≥75 years. MZL subtypes included extranodal (mucosa-associated lymphoid tissue) in 38.2%, nodal in 38.2%, splenic in 17.6%, and unknown in 5.9% of pts. The median number of prior therapies was 2 (range 1-6); 32.4% of pts had disease refractory to last therapy. Most (89.7%) pts received prior chemoimmunotherapy and 7 (10.3%) received rituximab monotherapy as their only prior treatment. Sixty-one (89.7%) pts had IRC-assessed FDG-avid disease.
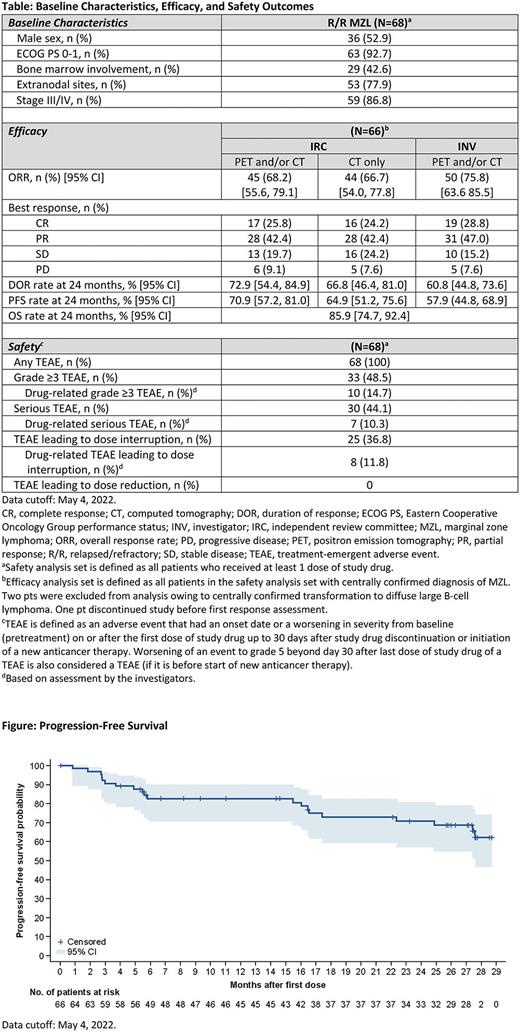
After a median follow-up of 28.0 months (range 1.6-32.9) and a median treatment duration of 24.2 months (range 0.9-32.9), 66 pts were evaluable for efficacy (Table). IRC-assessed ORR (complete response [CR] + partial response [PR]) was 68.2% (CR 25.8%). Responses were observed in all MZL subtypes, with an ORR of 64.0%, 76.0%, 66.7%, and 50.0% in extranodal, nodal, splenic, and unknown subtypes, respectively. CR rate was 40.0% for extranodal, 20.0% for nodal, 8.3% for splenic, and 25.0% for unknown subtypes. The median DOR, PFS, and OS were not reached. More than 70.0% of pts were alive or progression-free at the 2-year landmark by independent review (Figure).
Sensitivity analysis using only CT-based criteria (n=66) by IRC assessment showed an ORR of 66.7% and CR of 24.2%. Similarly, median DOR and median PFS were not reached.
At study completion, 31 (45.6%) pts deriving benefit rolled over to a long-term extension (LTE) study (NCT04170283); 24 (35.3%) pts discontinued owing to disease progression (investigator assessed); 5 (7.4%) due to adverse events (AEs), 2 (2.9%) required prohibited medications, and 1 (1.5%) withdrew consent.
The most common treatment-emergent AEs in >10% of pts were bruising (23.5%), diarrhea (22.1%), constipation (17.6%), arthralgia (14.7%), pyrexia (14.7%), upper respiratory tract infection (13.2%), abdominal pain and back pain (each 11.8%). Neutropenia (8.8%) and COVID-19 pneumonia (5.9%) were the most common grade ≥3 AEs. Five (7.4%) pts died due to unrelated AEs: COVID-19 pneumonia (n=2), acute myeloid leukemia (n=1, prior alkylating agent exposure), myocardial infarction (n=1, preexisting coronary artery disease), and septic encephalopathy (n=1, pt in CR). Hypertension occurred in 3 (4.4%) pts, atrial fibrillation and atrial flutter in 1 (1.5%) pt each; none led to treatment withdrawal. One (1.5%) pt experienced grade 3 gastrointestinal hemorrhage while receiving rivaroxaban for pulmonary embolism; pt fully recovered and rolled over to the LTE study.
Conclusion: In this final analysis with more than 2 years of median study follow-up, zanubrutinib continues to be effective as demonstrated by high response rates and durable disease control. Zanubrutinib was generally well tolerated, with no new safety signals observed.
Disclosures
Opat:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Consultancy; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Antengene: Consultancy; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding. Tedeschi:Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; Beigene: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau. Hu:Beigene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Research Funding. Linton:Genmab: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy; Beigene: Consultancy; Kite/Gilead: Consultancy; BMS/Celgene: Consultancy. McKay:Abbvie: Consultancy; AstraZeneca: Consultancy; Beigene: Consultancy; Celgene/BMS: Consultancy; Epizyme: Consultancy; Gilead/Kite: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Recordati Rare Diseases: Consultancy; Roche: Consultancy; Takeda: Consultancy. Chan:Roche: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Eusa: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees. Zinzani:Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Eusapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; University of Bologna: Current Employment. Browett:Eysa Pharma: Membership on an entity's Board of Directors or advisory committees; BeiGene: Research Funding; Arrowhead: Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding; MSD: Honoraria; Abbvie: Honoraria. Ke:BeiGene Ltd: Other: Travel, Accommodations, Expenses. Thieblemont:Roche: Consultancy, Honoraria, Other: Travel Support; Celgene: Consultancy, Honoraria, Other: Travel Support; Takeda: Consultancy, Honoraria, Other: Travel Support; Incyte: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support; Novartis: Consultancy, Honoraria, Other: Travel Support, Research Funding; AbbVie: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Other: Travel Support. Ardeshna:Gilead: Other; BMS: Other; Novartis: Other. Walker:BeiGene: Consultancy; Acerta: Consultancy. Hawkes:Merck KgA: Research Funding; Gilead: Honoraria; Merck Sharpe & Dohme: Honoraria; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; Roche: Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Honoraria, Other: Travel Fees, Speakers Bureau; Antigene: Honoraria; Novartis: Honoraria; BeiGene: Honoraria; Regeneron: Honoraria, Speakers Bureau; Specialised Therapeutics: Consultancy, Honoraria, Other; AstraZeneca: Research Funding, Speakers Bureau. Liang:BeiGene Ltd: Current Employment, Current equity holder in publicly-traded company, Other: Travel, Accommodations, Expenses. Xu:BeiGene US: Current Employment, Current equity holder in publicly-traded company; Janssen R&D: Ended employment in the past 24 months. Tankersley:BeiGene Ltd: Current Employment, Current equity holder in publicly-traded company, Other: Travel, Accommodations, Expenses. Delarue:BeiGene: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company. Co:BeiGene Ltd: Current Employment, Current equity holder in publicly-traded company, Other: Travel, Accommodations, and Expenses . Trotman:BMS, Roche, Janssen, Beigene, Cellectar,PCYC: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.